
NO2 Molecular Geometry / Shape and Bond Angles YouTube
Learn to determine if NO2 + is polar or nonpolar based on the polarity between bonds and the molecular geometry (shape).Ions, like NO2+ are sometimes confusi.
SOLVED Which molecule has polar bonds but is nonpolar? A. ClF3 B. H20
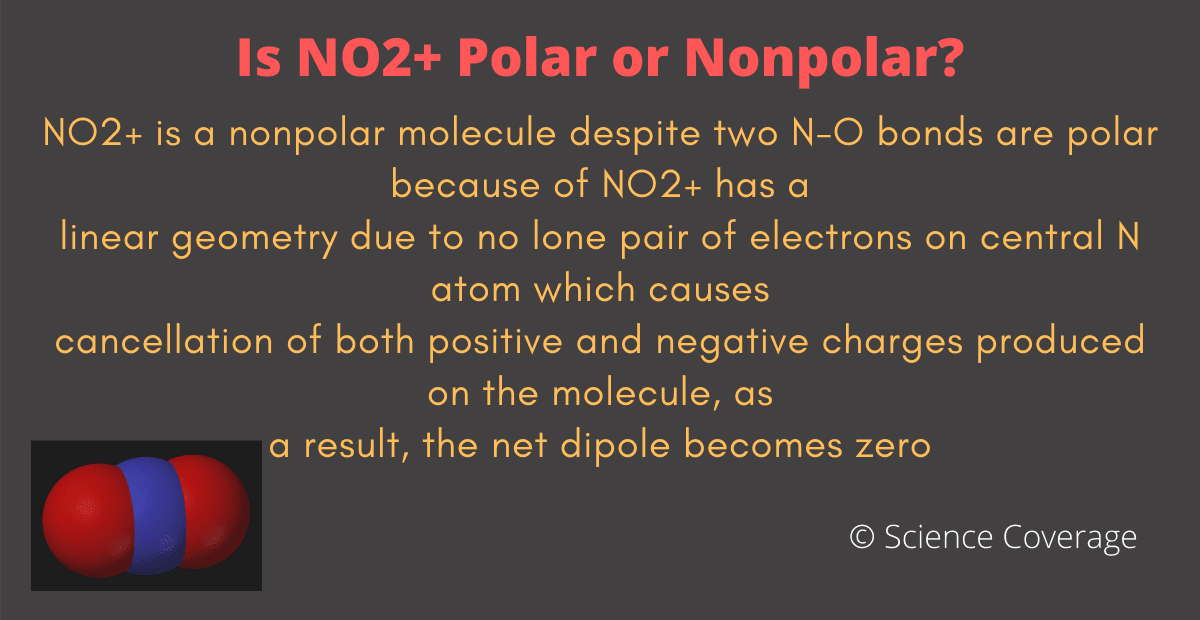
So, Is NO2+ Polar or Nonpolar? NO2+ (Nitronium ion) is nonpolar in nature because it has a linear geometrical structure due to which polarity of opposite NO bonds gets canceled by each other resulting in the nonpolar NO2+ ion. Nitronium ion is a stable ion in normal conditions.

Polar Bear No2
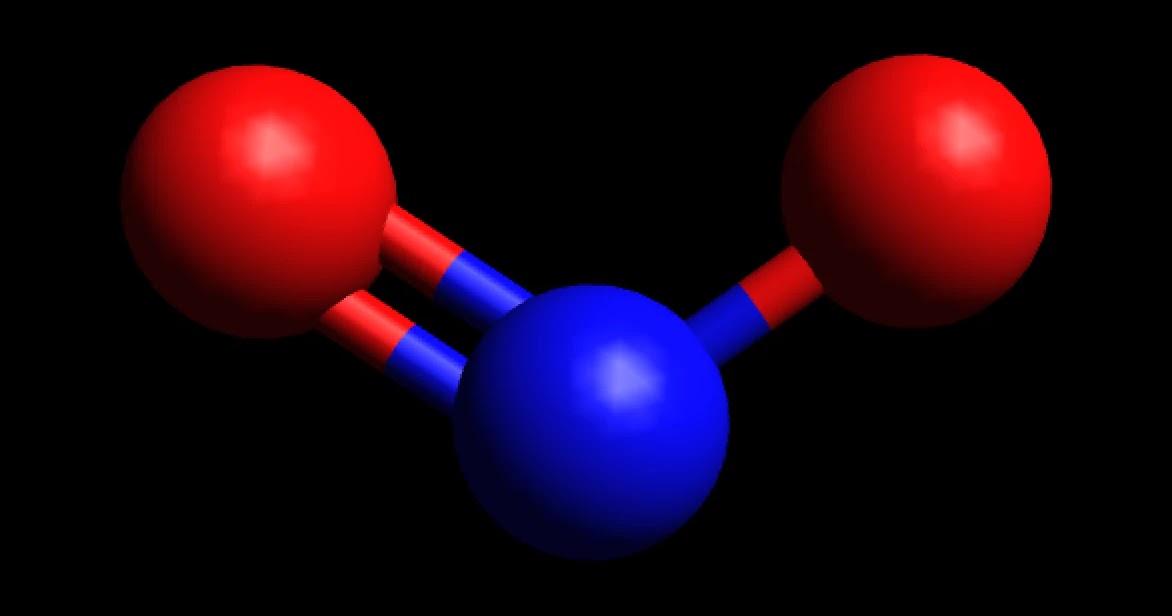
NO2 is a polar molecule and the Oxygen atom closest to negative side as the electronegativity of Oxygen (3.44) is comparatively greater than Nitrogen (3.04) so that Nitrogen has a partial positive charge and Oxygen has a partial negative charge established within the molecule.
MakeTheBrainHappy Is NO2 Polar or Nonpolar?
Carvone oxide, cis-Piperitenone oxide; Other names: trans-Carvone oxide; Carvone-1,2-epoxide; carvon-1,2-oxide; trans-Carvone epoxide; 1-methyl-4-(1-methylvinyl)-7-oxabicyclo[4.1.0]heptan-2-one Permanent link for this species. Use this link for bookmarking this species for future reference. Information on this page: Gas Chromatography.
[DIAGRAM] Dot Diagram Of N2o
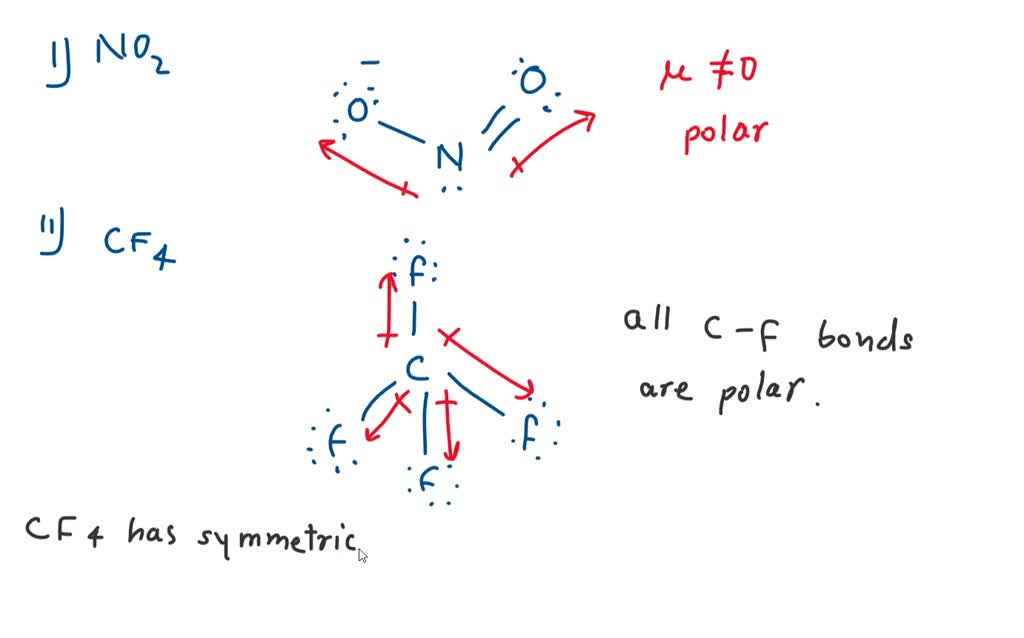
NO2 is a POLAR molecule because it has one unpaired electron on the Nitrogen atom (N) which causes the entire molecule to bend. This bending of NO2 molecule results in asymmetric geometry, which makes the molecule polar. Let me explain this in detail with the help of NO2 lewis structure and its 3D geometry. Why is NO2 a Polar molecule?

Is NO2+ Polar or Nonpolar? YouTube
Example 6.1.1 6.1. 1: Ele ctronegativity and Bond Polarity. Bond polarities play an important role in determining the structure of proteins. Using the electronegativity values in Figure 6.1.1 6.1. 1, arrange the following covalent bonds—all commonly found in amino acids—in order of increasing polarity.

No2 Lewis Structure
Learn to determine if NO (Nitric oxide) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structu.

Table of Contents
NO2 Polar or Nonpolar To determine if NO 2 is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. Nitrogen is the central atom, so we can draw the skeletal structure:

Is Nitrogen Dioxide (NO2) Polar or NonPolar? Lewis Structure YouTube
1. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Figure 4.12.1 4.12. 1 Some examples of nonpolar molecules based on molecular geometry (BF 3 and CCl 4 ). Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom.

Is NO2+ (Nitronium Ion) Polar or Nonpolar? YouTube
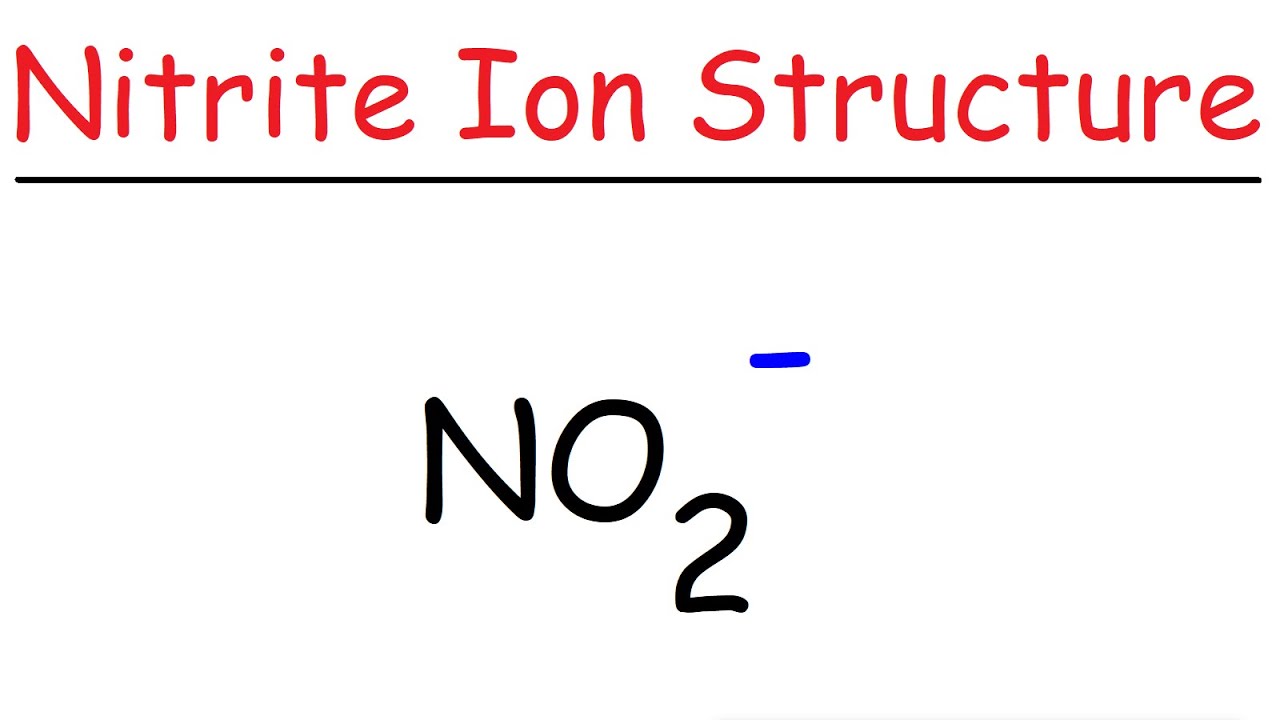
Nitrite [NO2]- is a polar molecular ion. It consists of a nitrogen (N) atom and two oxygen (O) atoms. The nitrogen atom is present at the center of the molecular ion, having a lone pair of electrons. In contrast, two oxygen (O) atoms occupy terminal positions, one on each side of the central N-atom, thus forming a bent shape.

NO2 é polar ou apolar
Table 13.5. 1: Bond Polarity. If the difference between the electronegativities of the two atoms is small, neither atom will take the shared electrons completely away from the other atom, and the bond will be covalent. This is the case when two nonmetals are bonded to each other.
Is NO2+ Polar or Nonpolar?
Is NO2 Polar or Nonpolar? (Nitrogen dioxide) Wayne Breslyn 724K subscribers Join Subscribe Subscribed 98 16K views 2 years ago Learn to determine if NO2 (Nitrogen dioxide) is polar or.

Is NO2 Polar Or NonPolar? NO2 Charge, NO2 Structure, NO2 Molar Mass
Who has not heard about NO2, Nitrogen Dioxide? It is one of the most common gaseous molecules having a reddish-brown hue. It can be cooled and compressed into a yellowish-brown liquid for tendshipping and transport. A highly toxic poisonous chemical compound, NO2 is a major air pollutant and belongs to the group of oxides of nitrogen.

2,154 Oxides Of Nitrogen Images, Stock Photos & Vectors Shutterstock
NO2+ is a nonpolar molecule despite two N-O bonds are polar. NO2+ has a linear geometry due to no lone pair of electrons on central N atom which causes cancellation of both positive and negative charges produced on the molecule, as a result, the net dipole becomes zero.
No2 Lewis Structure
Henry Agnew (UC Davis) 5.10: Electronegativity and Bond Polarity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Covalent bonds can be broken if energy is added to a molecule.

Draw Lewis structure for NO2^ + (nitronium ion).
Nitrogen dioxide is a chemical compound with the formula NO 2.One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas.It is a paramagnetic, bent molecule with C 2v point group symmetry.Industrially, NO 2 is an intermediate in the synthesis of nitric acid, millions of tons of which are produced each year, primarily for the production of fertilizers.